Exploring the role of readthrough-inducing molecule 2, 6-diaminopurine to increase immune response against cancer cells
Carmen Sandoval Pacheco, Alice M Leroy, Mehdi Derhourhi, Tristan Cardon, Catherine Leroy, Nathalie Jouy, Emmanuelle Com, Blandine Guevel, Roland Bourette, Julie Carrard, Daniela Barros, Belinda Duchêne, Bénédicte Toussaint, Philippe Froguel, Nicolas Jonckheere, Thierry Chassat, Isabelle Van Seuningen, Régis Lavigne, Charles Pineau, Philippe Pierre, Fabrice Soncin, Michel Salzet, Amélie Bonnefond, Fabrice Lejeune
Molecular Therapy
https://doi.org/10.1016/j.ymthe.2025.09.024
Abstract
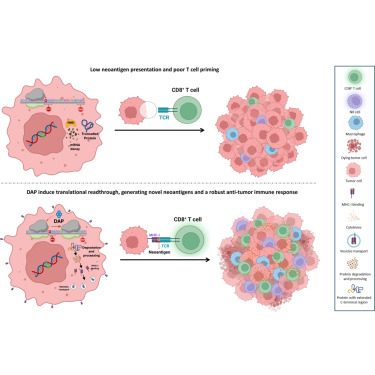
Immuno-oncotherapy is a highly promising therapeutic strategy that relies on the ability of cancer cells to present specific antigenic epitopes at their surfaces. Because they proliferate rapidly, cancer cells frequently accumulate genetic variants, including premature termination codons. In this study, we investigated the potential of 2,6-diaminopurine (DAP), a potent translational-readthrough-inducing molecule, to elicit an antitumor immune response. Readthrough-resulting proteins following DAP treatment can be displayed at the cell surface by the major histocompatibility complex, thus potentially enhancing immune recognition. This was demonstrated using a construct encoding FIREFLY LUCIFERASE interrupted by a UGA stop codon and fused at its C terminus with the SL8 antigenic peptide. Furthermore, in vivo exposure to DAP promotes the recruitment of immune effector cells, including T lymphocytes, macrophages, and natural killer cells, to the tumor microenvironment. These findings suggest that DAP and potentially other translational readthrough-inducing molecules hold promise as novel candidate drugs for antitumor therapy.
