Stratification of brain-derived extracellular vesicles of Alzheimer’s disease patients indicates a unique proteomic content and a higher seeding capacity of small extracellular vesicles
Marie Oosterlynck, Elodie Leroux, Balasubramaniam Namasivayam, Thomas Bouillet, Raphaelle Caillierez, Anne Loyens, Daniele Mazur, Romain Perbet, Christophe Lefebvre, Soulaimane Aboulouard, Claude-Alain Maurage, Bertrand Accart, Luc Buée, Morvane Colin
Translational Neurodegeneration
https://doi.org/10.1186/s40035-025-00519-z
Abstract
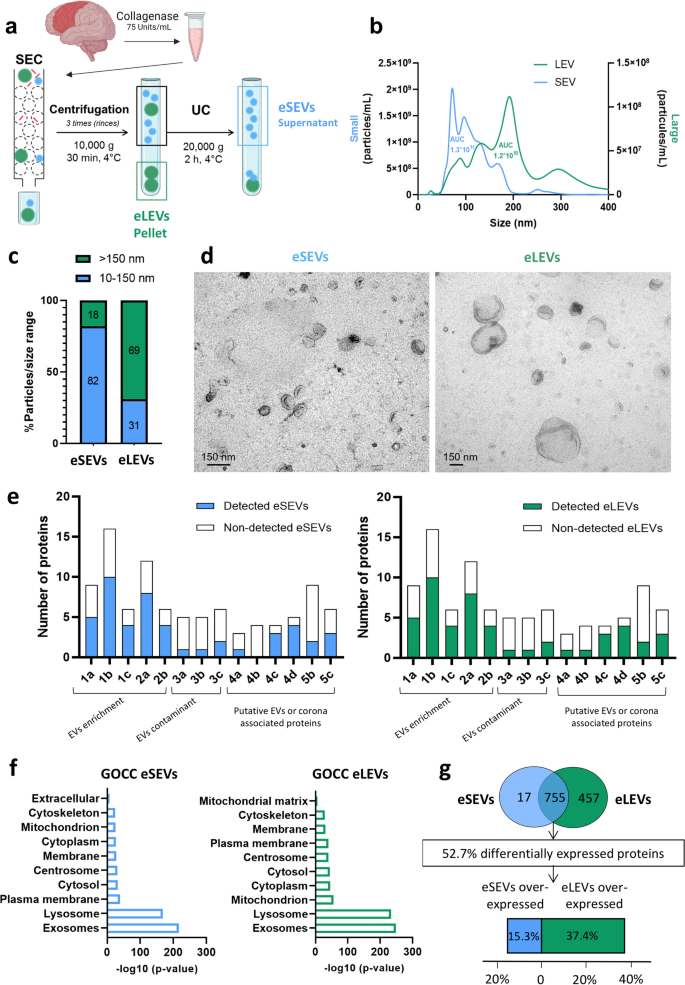
Background: Alzheimer’s disease (AD) is the most prominent form of dementia worldwide. It is characterized by tau lesions that spread throughout the brain in a spatio-temporal manner. This has led to the prion-like propagation hypothesis implicating a transfer of pathological tau seeds from cell to cell. Human brain-derived extracellular vesicles (BD-EVs) isolated from the brain-derived fluid of AD patients contain seeds that contribute to this tau pathology spreading. Knowing the rich diversity of EVs, isolation of functional EV sub-populations is required to unravel their implication in the pathophysiology of AD.
Methods: Here, enriched-small EVs (eSEVs) and enriched-large EVs (eLEVs) were isolated from frozen tissues after collagenase enzymatic brain dissociation to guarantee the best EVs’ integrity. Then proteomic profiling and tau seeding capacity testing were performed in vitro and in vivo.
Results: BD-EVs were stratified according to their size (eSEVs and eLEVs) and characterized to define new markers specific to EVs in AD. Both AD-derived eSEVs and eLEVs show the presence of GWAS-associated proteins and indicate a specific AD pathophysiological signature. Notably, AD eSEVs contain more proteins relative to the integrin-mediated synaptic signaling, while AD eLEVs proteins were more related to respiratory electron transport and brain immunity. Injection of these vesicles in transgenic mouse brain revealed that the AD-derived eSEVs are more prone than eLEVs to participate in the prion-like propagation and hence represent an interesting therapeutic target.
Conclusion: This study highlights the significant contribution of AD-derived EVs to tau propagation and provides new insights into different roles of EV sub-populations in AD.
